Abstract
- As the first audiovestibular medicine physician in the University of Port Harcourt Teaching Hospital, Nigeria, a keen observation revealed that a sizeable proportion of the adult patients presenting to our audiovestibular medicine clinic with vertigo are either known hypertensives, or hypertensives yet to have a formal diagnosis. This aroused an ardent curiosity to find the raison d’être, and to shed light on this pathological association between vertigo and hypertension. In line with this, it became imperative to highlight the dynamics of interaction, and pathophysiology behind the role of hypertension in vertigo.
-
Keywords: Hypertension; Vertigo; Audiovestibular; Cardiovascular diseases; Antihypertensive agents
INTRODUCTION
Vertigo is described as an illusion of spinning movement of the affected individual or his/her surrounding environment [1]. It occurs due to asymmetry in the coordination of the vestibular system, either from peripheral disorder or from disorder of its central connections [2]. The brain coordinates balance using inputs from the visual system, proprioception, and vestibule in the ear. The cerebellum and brainstem are pivotal in the central connections of these signals from the peripheral systems, and in the achievement of balance or stabilization of our body.
The autonomic nervous system plays a role in balance via central connections involving the hypothalamus and the thalamus, either through direct connection between these centers and the cerebellum and/or the vestibular nuclei [3,4], or by the effect of peripheral pathologic modulations of the body hemodynamics and autonomic functions [4]. As much as this may result in vertigo, it may often result in ‘dizziness,’ ‘loss of balance,’ ‘disequilibrium,’ ‘light-headedness’, or ‘presyncope’ that patients can refer to as vertigo.
Presyncopal dizziness occurs in patients with cardiovascular diseases, but some studies have reported it as vertigo due to cerebral ischemia in hypertensive patients [5]. However, Kim et al. [6] has reported that findings of isolated vertigo with underlying cardiac disease is referred to as ‘cardiogenic vertigo.’ This study highlighted features of cardiogenic vertigo, while proposing a diagnostic criterion [6]. There may be accompanying symptoms like chest discomfort, headache, palpitation, and light-headedness, but the most common cardiac abnormality during vertiginous attack in cardiogenic vertigo was bradyarrhythmia, which was seen in 89% of their study population [6] (Table 1).
The presence of underlying cardiac disease can help to differentiate cardiogenic vertigo from other vestibular disorders, but effort should be made to rule out the presence of other vestibular pathologies that may coexist in these patients. On the other hand, aging can cause neurodegenerative changes in the vestibule that can predispose to vertigo [7]. Therefore, in the presence of advancing age, comorbid diseases like diabetes mellitus, and hypertension can produce a synergistic pathologic effect that increases the predisposition to vestibulopathy, making these adult patients present with vertigo [8].
EPIDEMIOLOGY OF HYPERTENSION IN VERTIGO
In the study by Hendriks et al. [9], among the four subSaharan African countries studied (Nigeria, Tanzania, Kenya, and Namibia), Nigeria had the second highest crude prevalence of hypertension of 21.1% in its adult population in rural communities but had the least treatment rate of 5.1%. The prevalence of hypertension in vertigo/dizziness has been reported to be significant especially in the elderly population, but there is paucity of data on this subject matter in the sub-Saharan region. However, with the recent increased consciousness being created on this pathological association, and the robust interest in growing the subspecialty of audiovestibular medicine, research and data would increasingly emerge from the west African sub-region in the near future.
Hypertension can pose a quiet disorder due to its ability to remain unnoticed till it causes damage to target organs [10,11]. This can partly be responsible for some misdiagnosis of hypertension due to certain atypical presentations like vertigo, thus the World Health Organization has nicknamed hypertension “the silent killer” [10-12]. The association between hypertension and vertigo is such that in uncontrolled hypertension, vertigo, or dizziness can be the only symptom [1]. Several studies have reported significant proportion of cases of vertigo in patients with hypertension. The study carried out by Bunasuwan et al. [13] reported that 32.2% of patients who presented with vertigo in the otoneurologic clinic during their study in Thamassat Hospital in Thailand had underlying hypertension. Newman-Toker et al. [14] in the United States found that out of the 9,472 cases of dizziness, 21.1% were of cardiovascular origin. Lopes et al. [1] in Brazil studied 493 elderly subjects with vertigo, and 62.5% had been diagnosed of hypertension. Maarsingh et al. [15] worked on dizziness in family medicine clinic in Netherlands reported an incidence of dizziness as 47.1 per 1,000 person-years, and stated that in 40% of these patients, there was an independent association of dizziness with preexisting cardiovascular disease and preexisting hypertension.
PATHOPHYSIOLOGY OF HYPERTENSIVE VESTIBULOPATHY
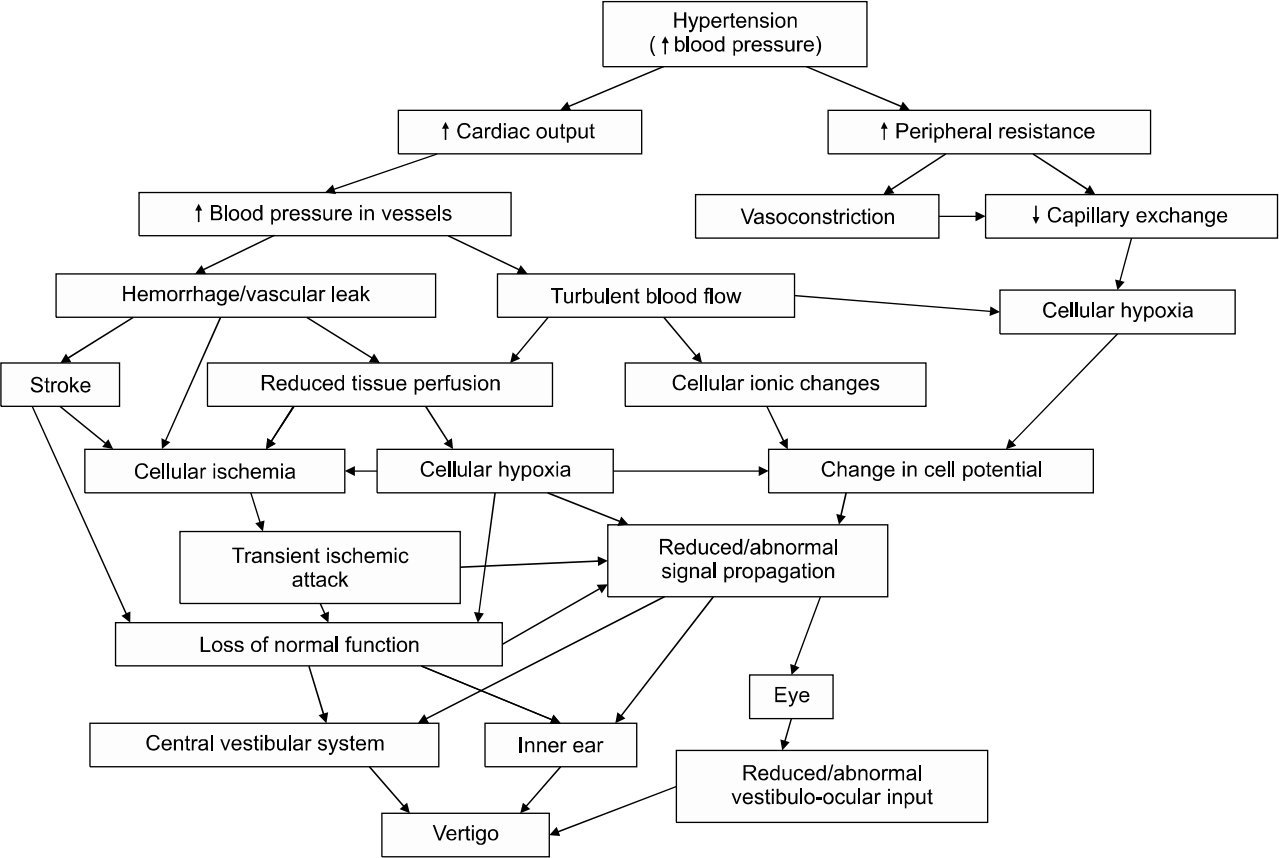
Hypertension is a common vascular disorder in adulthood, characterized by high blood pressure in the vascular system. This high blood pressure if not controlled, can lead to inner ear hemorrhage, or ischemia. When this involves the anterior inferior cerebellar artery (AICA), or the labyrinthine artery (only artery that supplies the inner ear) [16], it leads to a compromise of cochleovestibular function, and the patient can present with vertigo and/or hearing loss. This cardiovascular pathology can affect the vestibule in various ways. Firstly, hypertension can cause inner ear haemorrhage with its ischaemic effect on the vestibule and/or the cochlea [17]. Secondly, increase in blood viscosity, which reduces capillary permeability, with attendant reduced oxygen transport resulting in tissue hypoxia [16]. Finally, arterial hypertension can cause ionic changes that can affect cell potentials leading to modification in signal propagation [16,17]. Hypertension can affect both the central and the peripheral parts of balance, and these pathophysiological pathways can occur either in combination or in isolation to result in hypofunction or a complete failure of the vestibular system leading to vertigo (Fig. 1).
INNER EAR BLOOD SUPPLY
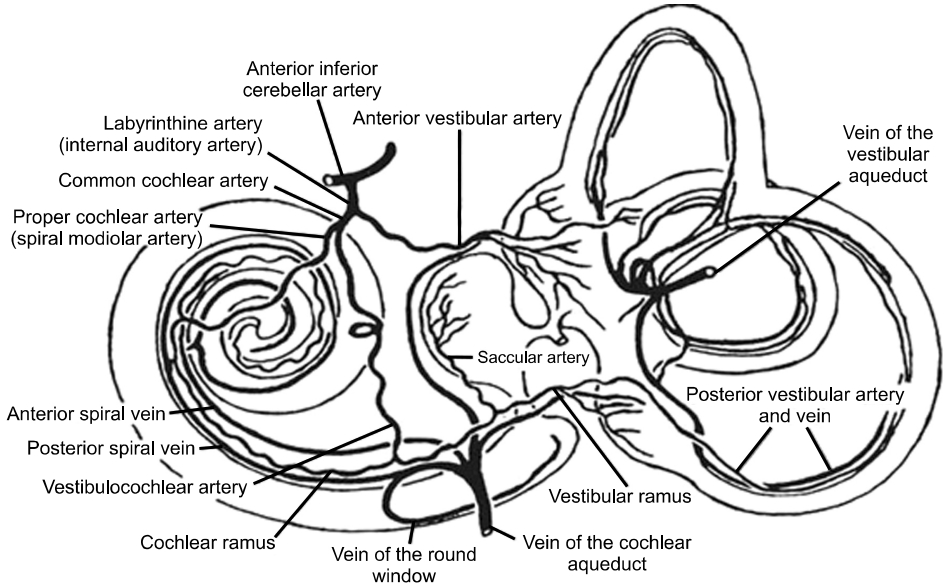
The blood supply to the labyrinth is only from the AICA, a branch of the basilar artery which is formed by the union of the left and right vertebral arteries. The labyrinthine artery which is a major branch of the AICA supplies the entire inner ear (Fig. 2 [18]). It divides into the common cochlear artery (CCA) and the anterior vestibular artery (AVA) [19]. The AVA runs with the superior vestibular nerve to supply the superior and horizontal semicircular canals, and the utricle, While the CCA bifurcates to form the cochlear artery and the posterior vestibular artery (PVA) [19,20]. The PVA supplies the posterior semicircular canals and the saccule, which are innervated by the inferior vestibular nerve.
The area supplied by the CCA is drained by the anterior vestibular vein, while the area supplied by the PVA is drained by the posterior vestibular. Other small veins from the semicircular canal join to form the veins of the vestibular aqueduct, which empties into the lateral venous sinus [19].
Hypertensive adults have a premature or accelerated onset of macro and micro vascular disease, as against the nonhypertensive adults [21], this makes them prone to microvascular complications [21] which may be coronary, cerebral or a peripheral vascular disease, which can involve various parts of the body but highlighted in the sensory organs [22]. The pattern of inner ear blood supply described above makes it apparent that microvascular complications can compromise vascular integrity and threaten labyrinthine function, irrespective of the exact underlying etiopathogenesis [23,24]. In other words, disruption, or alteration in the blood supply to the vestibular labyrinth by ischemia or infarction can cause vertigo, disequilibrium, blurred vision, and nausea. However, if the labyrinthine artery is affected prior to its bifurcation, then hearing loss is more likely to occur [25].
VERTIGO IN UNCONTROLLED HYPERTENSION
The effect of uncontrolled hypertension usually results in vascular aneurysms, and/or microvascular hemorrhage with its ischemic effect. Vasopressin release by the pituitary gland usually occurs in response to a change in blood volume and pressure, initiated by sensitization of the arterial baroreceptors and arterial stretch receptors [4,26]. However, in uncontrolled hypertension, these arterial stretch receptors are overwhelmed by a continuous high blood pressure, which over time, erodes the body’s ability to self-regulate changes in blood pressure [27]. This failed autoregulation makes subjects susceptible to the complications of hypertension, and when the inner ear vasculature is affected, patient can present with vertigo and/or hearing loss [23]. This effect of uncontrolled hypertension leads to damage to both the cerebral vasculature and to the inner ear vasculature, leading to both central and peripheral causes of vertigo. The systematic review by Shekhar et al. [27] showed that chronic uncontrolled hypertension can result in dysfunction of the cerebral autoregulation leading to a leakage of the blood brain barrier [27,28]. This causes infiltration of the brain by inflammatory factors leading to glial activation and subsequent inflammatory and ischemic injury [27,28]. When this affects the areas of the brain that are involved in vestibular network, it can result in neuronal death and synaptic dysfunction in the propagation of vestibular signals, leading to vertigo [29,30].
The effect of uncontrolled hypertension can lead to a cerebrovascular accident also known as stroke. A stroke is often preceded by the formation of an aneurysm on a brain vessel, increasing its susceptibility to rupture and hemorrhage into the brain [31]. Berry aneurysm which is a common complication of chronic uncontrolled hypertension increases the risk of stroke in a patient, likewise other aneurysms like the Arnold Chiari malformation, or aneurysms on blood vessels supplying any part of the central vestibular system can lead to vertigo [32,33]. When this effect of uncontrolled hypertension involves the cerebellar vessels, like the AICA or the posterior inferior cerebellar artery (PICA), then an AICA stroke or PICA stroke will occur. This has been shown to cause vertigo due to vascular compromise of the cerebellum and/or the inner ear [25]. This is because the labyrinthine artery which supplies the inner ear is an aberrant artery that usually comes from the AICA but may emanates from the PICA which is a branch of the vertebral artery [19,34]. The cerebellar compromise from the leaking of either of the AICA or PICA can cause vertigo, but the added effect of the inner ear compromise due to the involvement of the labyrinthine artery implies the presence of signs of peripheral vertigo and in some cases hearing loss. In these cases, the Head Impulse, Nystagmus, Test of Skew (HINTS) exam becomes a very useful bedside examination for diagnosis.
Besides the direct effect of uncontrolled hypertension on the inner ear vasculature, vertigo can result due to the effect of hypertension on the eyes. Vision contributes 70% of peripheral input required for balance, as compared to 25% from the vestibular and 5% from proprioception, and dominates both the feeling of self-movement, and postural control [26]. Studies have reported a three-fold increase in sway when patients have their eyes closed during vestibular examinations, buttressing how critical vision is to balance [26]. The ability of hypertension to impair vision due to retinopathy can elucidate how much uncontrolled hypertension can affect balance, and the importance of eye examination in hypertensive patients presenting with vertigo. As much as uncontrolled hypertension can cause vertigo, the hypotensive effect of its treatment in the bid to achieve a good blood pressure control can also pose a vertiginous challenge if not well coordinated [35,36], thus making close and adequate monitoring of blood pressure control during treatment very crucial.
VERTIGO IN NEUROVASCULAR PATHOLOGY
Neurovascular or cerebrovascular injuries usually can present as stroke, with acute dizziness. Acute dizziness or vertigo are usually of otovestibular origin, such as in the benign paroxysmal positional vertigo (BPPV), vestibular neuritis, Menière’s disease, and labyrinthitis, but can be a symptom indicating an impending stroke [31,37]. Stroke is a common misdiagnosis which has been identified as the 4th most common diagnostic error, with frequency of misdiagnosis of about 13% [31]. This is because only about 20% of patients with acute stroke had focal neurologic deficit [14,31]. Stroke diagnosis is particularly challenging in a patient presenting with dizziness in the absence of neurological deficit [25]. Inability to recognize acute stroke may result in a poor patient outcome, so a high index of suspicion is required on the part of the clinician. Clinical tests like the HINTS exam can identify stroke, especially in cases of cerebellar stroke [25]. Several clinical or bedside oculomotor and neurological examinations, including Dix-Hallpike, head thrust, and HINTS+, can be useful and reliable in predicting focal neurological abnormalities when carried out by a trained clinician [25,31]. This makes such clinical examinations crucial in vertigo patients.
Vertebrobasilar insufficiency due to vertebrobasilar transient ischemic attack has been implicated in transient recurrent vertigo in the absence of inner ear, brainstem, or cerebellar disorder [36,38]. The cause is attributable to a decrease in cerebral blood flow due to hypotension, which leads to reversible functional changes, and this effect of a transient, reversible impairment in vertebrobasilar blood flow, may not be obvious on audiometric and vestibular tests [32,39]. However, in continued deterioration of blood flow or damage due to persisting hypotension, functional and anatomical changes may become obvious [40]. Audiometric findings in these patients when obvious, are usually in the low frequency sounds, and often patient may not be aware of its presence [36,38,39,41,42].
VERTIGO IN ORTHOSTATIC (POSTURAL) HYPOTENSION
Orthostatic hypotension is defined as “a sustained reduction of systolic blood pressure of at least 20 mmHg or diastolic blood pressure of 10 mmHg within 3 minutes of standing up or during head-up tilt test, frequently accompanied by dizziness, fainting and other symptoms that presents on rising from a sitting to standing position, or from lying to a sitting/standing position” [43]. It can occur as a symptom of dysautonomia, or secondary to cardiovascular diseases, anaemia or other pathologies that can affect the autonomic system and/or alter the haemodynamics balance. Postural tachycardia syndrome can similarly cause orthostatic dizziness/vertigo [43,44]. The term orthostatic dizziness/vertigo is not appropriate if symptoms occur while patient is in supine position [45]. The normal response to orthostasis is such that there is a hormone reaction which affects the renin-angiotensin-aldosterone system, leading to sodium and water retention to increase blood volume, in addition to the release of other peptides and active neuroamines like vasopressin and epinephrine, and the effect of their action is obvious within minutes of orthostasis [26]. However, in these patients with orthostatic hypotension, there is a failure of this peripheral autoregulatory mechanism, which leaves them vulnerable to the effects of the sustained hypotension such as dizziness [5,44].
As much as cardiovascular diseases causing microangiopathy has been implicated in labyrinthine pathologies, orthostatic hypotension is common in the elderly patients especially in the presence of coexisting diabetes mellitus [46]. These symptoms of vertigo may also occur in young adults even in the absence of cardiovascular risk factors [35]. Soto and Vega [30] reported that the vestibular system functions in such a way that the blood brain barrier isolates the central vestibular system, while the vestibule is isolated from the peripheral circulation by the blood-labyrinthine barrier. Thus, drugs can affect the peripheral system without affecting the central system, or vice versa. The benefit of this phenomenon is in the administration of local pharmacological agents in the ear, without the affectation of the central vestibular system [39,47]. Hypotension which results due to abnormal vasomotor regulation may generate a transient ischemia that can negatively affect cochleovestibular perfusion [24]. However, hypotension without a corresponding change in vasomotor reactivity is not usually sufficient to cause inner ear damage [48]. The reduced autoregulation of the cochleovestibular circulation poses a greater risk of vertigo, as compared to cerebral circulation [24,49]. In other words, this greater risk of inner ear damage is because the brain has a more efficient autoregulatory mechanism than the blood-labyrinthine barrier [24,49].
Some studies have reported that the dizziness or vertigo seen in orthostatic hypotension is due to a reduction in cerebral blood flow, especially the vertebral artery [32,41], while others have reported that vertigo is due to a failure of inner ear prefusion especially to the vestibule [23,35,50]. However, in the face of recent evidence, vertigo can be attributed to a combined hypoperfusion effect of both the central and peripheral aspects of the vestibular system, with a concomitant effect of the negative hemodynamic and autonomic outcomes [51]. Some studies have stated that the exact pathophysiology through which hypotension causes vertigo may still be poorly understood [44,51]. Irrespective of what the exact pathophysiological mechanism of orthostatic hypotension in vertigo may be, it has been shown to play a very important role in the cause of vertigo in patients with hypertension. Sequel to the above findings, any decision to make the estimation of blood pressure while in sitting, lying, and standing position compulsory for every hypertensive patient presenting with vertigo to any audiovestibular medicine clinic may be a valuable way of ruling out or identifying this important predisposition to vertigo in hypertension. However, when assessing the functional status and risk of fall and syncope in these patients, assessment of dizziness on standing becomes more beneficial than just the measurement of postural blood pressure change. A concerted multidisciplinary approach between the cardiologist and audiovestibular medicine physician may be the way forward in the management these patients.
VERTIGO IN CARDIAC INSUFFICIENCY
However imprecise, vertigo is a common symptom in cardiac disease. Though patients may use the term ‘vertigo’ to describe any feeling of ‘fainting,’ ‘imbalance,’ ‘disequilibrium,’ ‘light-headedness,’ or ‘spinning,’ the important clinical task is in identifying underlying disease and to implement a sound clinical decision [2]. Clinicians should be clinically aware that vertigo can present as an indication to the presence of cardiac disease or its complication [2]. Cardiac insufficiency which encompasses both heart valvular disease and cardiomyopathies can cause vertigo not only from the inability of the heart to pump enough blood to keep up with the normal blood pressure in the blood vessel but also in failure of the heart muscles to keep up with force needed to achieve optimum circulation. Cardiac insufficiency can also be due to chronic negative effect on aortic and carotid bodies, vasoreceptors and baroreceptors which over time fail in their ability to regulate vasomotor autonomic function [39].
There are four heart valves that ensure blood flows in the right direction in the heart; they include the mitral, tricuspid, aortic, and pulmonary valves. They open and close at specific times during heartbeat, but when its opening and closing fails to occur as normal, for instance due to regurgitation, stenosis, or atresia, it impairs blood flow to the body [52]. Heart valve disease may not present with symptoms for a while, but when it does, vertigo can be one of its symptoms [52]. For instance, dizziness has been reported following mitral valvular repair [53]. This could be attributed to standing up too quickly, or a symptom of heart failure, it can also occur due to abnormal heart function or heart rhythm from narrowed valve. Most treatments for heart failure like digitalis glycosides, diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, and beta blockers affect blood pressure, and despite improvement in heart function, dizziness can occur due to their blood pressure lowering effect, especially when standing up from a sitting or lying position. On the other hand, in patients with underlying cardiac disease where vertigo is the only presenting symptom, a careful evaluation of history and clinical examination may expose a predisposition to or clinical evidence of hypertension or heart disease [54,55].
VERTIGO AND ANTIHYPERTENSIVE THERAPY
Antihypertensive therapy has been implicated in vertigo, falls, orthostatic hypotension, and syncope in elderly adults. Several studies have shown dizziness or vertigo as a side of effect of antihypertensive therapy [56,57], however, whether one class of antihypertensive causing vertigo more than the other classes have remained controversial [15,57]. Medication induced dizziness can be difficult to identify especially in elderly persons in whom it can be masked by the changes associated with aging [15,57]. Despite that vertigo is prevalent in adults aged 65 years and above, normal aging does not cause vertigo [58]. Factors like comorbid diseases and their complications [59], drug-related problems and polypharmacy predispose these elderly persons to vertigo [58-60]. The various classes of drugs used in high blood pressure and their effects are shown in Table 2.
Pirodda [35] argued in his study that the ability of antihypertensives to affect labyrinthine function and cause vertigo or hearing loss, is in the hypotension they generate. The study confirmed that a rapid lowering of blood pressure, followed by a sharp vasoconstriction is what threatens the cochlea-vestibular system. In addition, because of the terminal blood supply pattern of the inner ear, which mimics a typical model of end organ, it is prone to an acute lack of perfusion, with a resultant degenerative effect [61].
Antihypertensive monotherapy has been associated with less side effects of dizziness when compared to combination therapy. However, it has been found that some classes of antihypertensive agents have been reported to be associated with more side effect of vertigo, due to their ability to cause a more rapid blood pressure lowering effect [62]. Levodopa has been associated with significant orthostatic hypotension, due to its ability to reduce blood pressure via different mechanism [63,64], while diuretics have been associated with both vertiginous and hearing loss side effects [56]. Likewise, ACE inhibitors have also been reported to have significant dizziness as side effect [57,64]. Studies have also shown a higher prevalence of dizziness in females using antihypertensives than in their male counterparts, particularly in women using propranolol alone, diuretics alone, and diuretics in combination with any other agent like hydralazine, clonidine, and reserpine [57].
Combination antihypertensive therapy has been found to cause dizziness more than monotherapy. For instance, Chimirri et al. [65] asserted that antihypertensive treatment using amlodipine and irbesartan-hydrochlorothiazide combination causes significant vertigo. Similarly, adverse reaction rate of telmisartanamlodipine combination in antihypertensive therapy was 7.69% with headache and vertigo as commonest side effects [66]. The study by Juraschek et al. [57] on the effect of antihypertensive class on falls, syncope and orthostatic hypotension; the ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) showed that a more intensive antihypertensive therapy was associated with a greater risk of syncope, but not with falls, or orthostatic hypotension. Syncope or presyncope has been identified as one of the feelings patients can describe as vertigo. Combination of beta blocker with a calcium channel blocker caused serious dizziness in patients that necessitated withdrawal from treatment [67]. However, this was found to be dose-dependent, and agent specific. Combinations of beta blocker with verapamil or diltiazem was found to be more efficacious in treatment, and at the same time, associated with higher rate of dizziness [68,69]. Thus, clinicians need to consider both the combination of choice of the calcium channel blocker, and the dose of the calcium channel blocker and beta blocker when deciding on specific anti-hypertensive treatment for a patient [68].
The versatility of antihypertensives that have been associated with dizziness specifically in combination therapy [64-69], implies the need for the cardiologist to be adept to the side effects of various antihypertensive agents, mostly as regards the dosedependent, and agent-specific nature of these side effects. In addition, clinicians must consider a preexisting conduction system disease, and the possibility of drug interactions [66]. Despite the crucial role of combination antihypertensive therapy in the management of patients resistant to single drug treatment, the possibility of a greater adverse effect should buttress the importance of a careful patient selection and close clinical monitoring [57].
ROLE OF HYPERTENSION IN VERTIGO: THE WAY FORWARD
Vertigo is a common presenting complain in adults seen in the audiovestibular clinic, especially in the elderly population, and has been associated with comorbid diseases like hypertension, and medications like antihypertensive agents. For clinicians to understand and appropriately manage vertigo, there must be a good understanding of the underlying factors/pathologies that may be responsible for vertigo in these patients [70], in line with a high index of suspicion of the pathological association between hypertension and vertigo. This is necessary in the assessment of the dizzy patient, and in ensuring a precise clinical decision and management [57]. In line with this pathological association, the cardiologist or the audiovestibular physician cannot manage such patients in isolation. It requires a wholistic approach via a practicable form of co-management to manage these patients. However, judging from the fact that majority of vestibular disorders are peripheral in origin [70,71], the audiovestibular physician plays a primary role of excluding these peripheral causes of vertigo in the management of these patients.
CONCLUSION
When hypertensive patients present with vertigo in clinic, focus should be in finding and addressing underlying pathology. It may be safe to infer that vertigo when present in a patient with hypertension, indicates either a poorly controlled blood pressure, presence of complications in hypertension, or a poorly coordinated antihypertensive therapy. Therefore, this pathological association when present, should be a reveille for a concerted co-management effort between the cardiologist, the audiovestibular medicine physician, and the neurologist in some cases, to maintain an equilibrium that will ensure the best outcome in these adult patients with hypertension.
RECOMMENDATION
Presence of a raised blood pressure, or a history of hypertension, should raise an index of suspicion in clinicians for this pathological association between hypertension and vertigo. In addition, effort should be made to rule out cardiovascular and/or neurovascular causes in such patients. Finally, a co-management strategy should be adopted involving the cardiologist, the audiovestibular medicine doctor and when necessary, the neurologist.
ARTICLE INFORMATION
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING/SUPPORT
None.
Fig. 1.Schematic representation of hypertensive vestibulopathy.
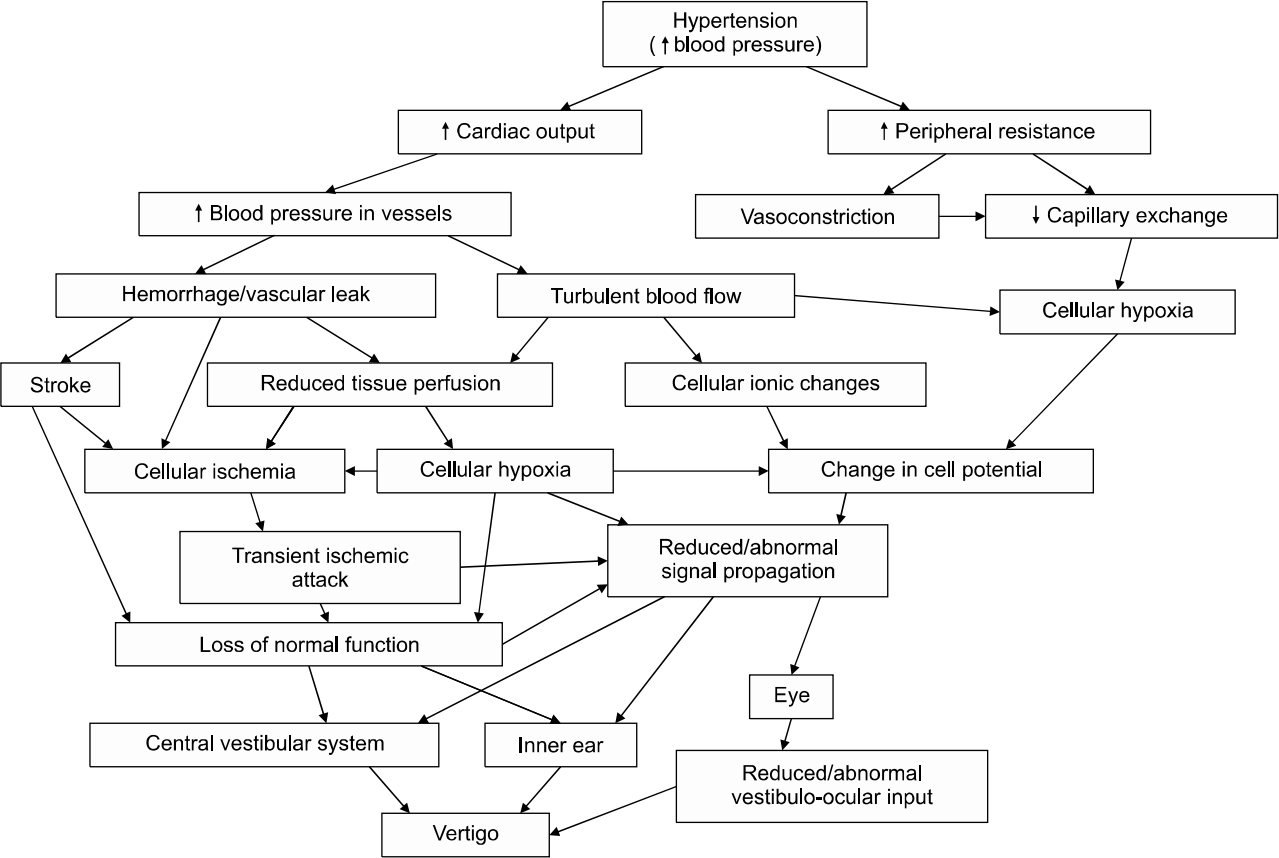
Fig. 2.Diagram of blood supply to the inner ear. Reprinted from the article of Lysakowski [18], according to the Creative Commons License.
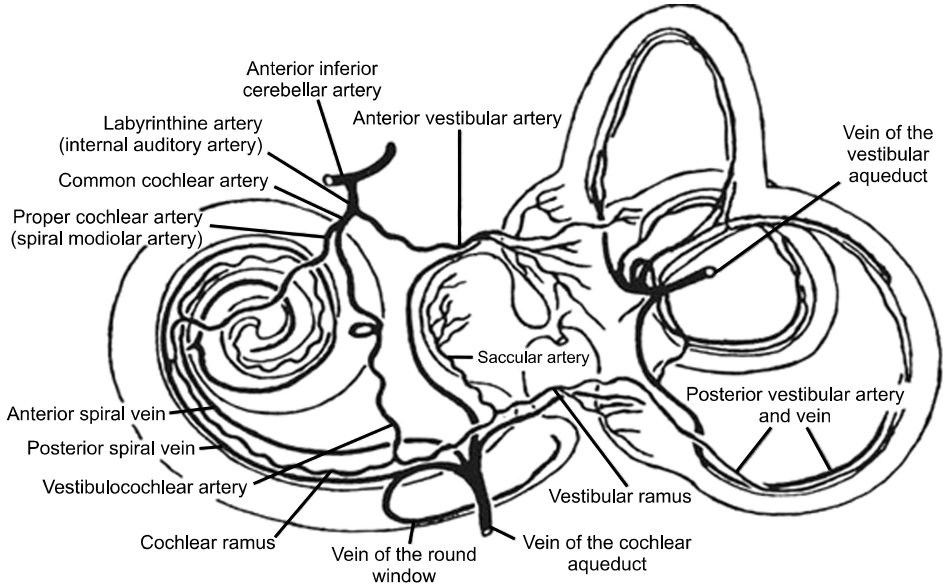
Table 1.Proposed diagnostic criteria for cardiogenic vertigo
|
Definite Cardiogenic vertigo (every point needs to be present) |
|
|
a) Recurrent attacks of spontaneous spinning or non-spinning vertigo |
|
b) Onset at ≥60 yr of age |
|
c) Duration of vertigo ≤1 min |
|
d) Documentation of cardiac disease during an attack of vertigo |
|
e) Response to or improvement with proper treatment for cardiac diseases |
|
f) Not better accounted for by another diagnosis |
|
Probable cardiogenic vertigo (each point needs to be fulfilled) |
|
a) More than one attack of spontaneous spinning or non-spinning vertigo |
|
b) Duration of vertigo ≤5 min |
|
c) Cardiac disease documented |
|
d) Not better accounted for by another diagnosis |
|
d) Not better accounted for by another diagnosis |
Table 2.Classes of drugs used in hypertension and their effects
|
Class of medication |
Examples |
Effect of medication |
|
Angiotensin-converting enzyme (ACE) inhibitors |
Captopril, lisinopril |
Hypotension |
|
Beta blockers |
Propranolol, nadolol |
Hypotension |
|
Calcium channel blockers |
Nifedipine, verapamil |
Vasodilation, hypotension |
|
Peripherally acting alpha adrenergic blockers |
Reserpine |
Hypotension |
|
Angiotensin II antagonists/angiotensin II receptor blockers (ARBs) |
Valsartan, losartan |
Hypotension |
|
Vasodilators |
Hydralazine, minoxidil |
Hypotension, vasodilation |
|
Centrally acting alpha adrenergic blockers |
Prazosin, doxazosin |
Hypotension, bradycardia |
|
Diuretics |
Spironolactone, furosemide |
Vasodilation, volume contraction |
|
Central agonists |
Levodopa, methyldopa |
Hypotension |
|
Lipid-lowering agents |
Statins |
Hypotension |
|
Digitalis glycoside |
Digoxin |
Hypotension |
REFERENCES
- 1. Lopes AR, Moreira MD, Trelha CS, Marchiori LL. Association between complaints of dizziness and hypertension in non-institutionalized elders. Int Arch Otorhinolaryngol 2013;17:157–62.ArticlePubMedPMC
- 2. M uncie HL, Sirmans SM, James E. Dizziness: approach to evaluation and management. Am Fam Physician 2017;95:154–62.PubMed
- 3. Ruffle JK, Hyare H, Howard MA, Farmer AD, Apkarian AV, Williams S, et al. The autonomic brain: multi-dimensional generative hierarchical modelling of the autonomic connectome. Cortex 2021;143:164–79.ArticlePubMedPMC
- 4. Takeda N. Autonomic dysfunction in patients with vertigo. JMAJ 2006;49:153–7.
- 5. Choi JH, S eo JD, Kim MJ, Choi B Y, Choi Y R, Cho B M, et al. Vertigo and nystagmus in orthostatic hypotension. Eur J Neurol 2015;22:648–55.ArticlePubMed
- 6. Kim HA, A hn J, Park HS, Lee SM, Choi SY, Oh EH, et al. Cardiogenic vertigo: characteristics and proposed diagnostic criteria. J Neurol 2021;268:1070–5.ArticlePubMedPDF
- 7. Osuji AE. Classification, pathophysiology, genetics, and role of lifestyle medicine in presbycusis. scholarly journal of otorhinolaryngology. Sch J Otorhinolaryngol 2021;6:638–43.
- 8. Osuji AE, Da Lilly-Tariah OB, Unachukwu CN, Nwankwo BE. Comparison of hearing threshold in hypertensive and non hypertensive type 2 DM. J Otorhinolaryngol Disord Treat 2020;1:1–7.
- 9. Hendriks ME, Wit FW, Roos MT, Brewster LM, Akande TM, de Beer IH, et al. Hypertension in sub-Saharan Africa: crosssectional surveys in four rural and urban communities. PLoS One 2012;7:e32638. ArticlePubMedPMC
- 10. Singh R, Sinha RK, Mani C, Singh R, Pal R. Burden and vulnerability of hypertension in a rural population of Patna, Bihar, India. South East Asia J Public Health 2013;1:53–8.ArticlePDF
- 11. World Health Organization (WHO). A global brief on hypertension [Internet]. Geneva, WHO. 2013;[cited 2022 Jan 25]; Available from: https://www.who.int/publications/i/item/a-globalbrief-on-hypertension-silent-killer-global-public-health-crisisworld-health-day-2013.
- 12. Stevens KN, Lang IA, Guralnik JM, Melzer D. Epidemiology of balance and dizziness in a national population: findings from the English Longitudinal Study of Ageing. Age Ageing 2008;37:300–5.ArticlePubMed
- 13. Bunasuwan P, Bunbanjerdsuk S, Nilsuwan A. Etiology of vertigo in Thai patients at Thammasat Hospital. J Med Assoc Thai 2011;94 Suppl 7:S102–8.PubMed
- 14. Newman-Toker D E, Hsieh YH, Camargo CA Jr, Pelletier AJ, Butchy GT, Edlow JA. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc 2008;83:765–75.ArticlePubMedPMC
- 15. Maarsingh OR, Dros J, Schellevis FG, van Weert HC, Bindels PJ, Horst HE. Dizziness reported by elderly patients in family practice: prevalence, incidence, and clinical characteristics. BMC Fam Pract 2010;11:2. ArticlePubMedPMCPDF
- 16. de Moraes Marchiori LL, de Almeida Rego Filho E, Matsuo T. Hypertension as a factor associated with hearing loss. Braz J Otorhinolaryngol 2006;72:533–40.ArticlePubMed
- 17. Agarwal S, Mishra A, Jagade M, Kasbekar V, Nagle SK. Effects of hypertension on hearing. Indian J Otolaryngol Head Neck Surg 2013;65(Suppl 3):614–8.ArticlePubMedPMCPDF
- 18. Lysakowski A. Anatomy of the vestibular system [Internet]. Ento Key. 2016;[cited 2022 Mar 21]. Available from: https://entokey.com/anatomy-of-the-vestibular-system/.
- 19. Cohen HS. Vestibular rehabilitation and stroke. In: Gillen G editors. Stroke rehabilitation. St. Louis (MS): Elsevier; 2016. p.416–23.
- 20. Vestib.com. Arterial supply to the structures of balance [Internet]. Vestib.com. c2013;[cited 2022 Feb 2]. Available from: https://vestib.com/arterial-supply.html.
- 21. Odum EP, Orluwene CG. Metabolic syndrome prevalence in healthy individuals in University of Port Harcourt Teaching Hospital (Upth), Port Harcourt. IOSR-JDMS 2013;10:17–22.Article
- 22. Colledge NR, Walker BR, Ralston Stuart, Davidson A. Davidson’s principles and practice of medicine. Edinburgh, New York: Churchill Livingstone/Elsevier; 2010.
- 23. Juhn SK, Hunter BA, Odland RM. Blood-labyrinth barrier and fluid dynamics of the inner ear. Int Tinnitus J 2001;7:72–83.PubMed
- 24. Nakashima T. Autoregulation of cochlear blood flow. Nagoya J Med Sci 1999;62:1–9.PubMed
- 25. Tarnutzer AA, Berkowitz AL, Robinson KA, Hsieh YH, Newman-Toker DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011;183:E571–92.ArticlePubMedPMC
- 26. Acar S, Demırbüken İ, Algun C, Malkoç M, Tekın N. Is hypertension a risk factor for poor balance control in elderly adults? J Phys Ther Sci 2015;27:901–4.ArticlePubMedPMC
- 27. Shekhar S, Liu R, Travis OK, Roman RJ, Fan F. Cerebral autoregulation in hypertension and ischemic stroke: a mini review. J Pharm Sci Exp Pharmacol 2017;2017:21–7.PubMedPMC
- 28. Wollner L, McCarthy ST, Soper ND, Macy DJ. Failure of cerebral autoregulation as a cause of brain dysfunction in the elderly. Br Med J 1979;1:1117–8.ArticlePubMedPMC
- 29. Baloh RW. Vertebrobasilar insufficiency and stroke. Otolaryngol Head Neck Surg 1995;112:114–7.ArticlePubMed
- 30. Soto E, Vega R. Neuropharmacology of vestibular system disorders. Curr Neuropharmacol 2010;8:26–40.ArticlePubMedPMC
- 31. Kim JS, Bae HJ, Kim M, Ahn S, Sohn CH, Seo DW, et al. Stroke prediction in patients presenting with isolated dizziness in the emergency department. Sci Rep 2021;11:6114. ArticlePubMedPMCPDF
- 32. Matsunaga T. Correlation between vertigo and abnormal hemodynamics of the vertebral artery. Practica Oto-Rhino-Laryngologica 1992;85:1531–41.Article
- 33. Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959;39:183–238.ArticlePubMed
- 34. Eggers SD, Zee D. Vertigo and imbalance clinical neurophysiology of the vestibular system handbook of clinical neurophysiology. Amsterdam: Elsevier; 2009.
- 35. Pirodda A. Antihypertensive drug and inner ear perfusion: an otologist's point of view. Pharmaceuticals (Basel) 2009;2:44–8.ArticlePubMedPMC
- 36. Kubo T. Dizziness due to cerebrovascular disease. Equilibrium Res 1991;50:339–45.Article
- 37. Neuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, et al. Epidemiology of vestibular vertigo: a neurotologic survey of the general population. Neurology 2005;65:898–904.ArticlePubMed
- 38. Williams D, Wilson TG. The diagnosis of the major and minor syndromes of basilar insufficiency. Brain 1962;85:741–74.ArticlePubMed
- 39. Fisher CM. Vertigo in cerebrovascular disease. Arch Otolaryngol 1967;85:529–34.ArticlePubMed
- 40. Reed G, Devous M. Cerebral blood flow autoregulation and hypertension. Am J Med Sci 1985;289:37–44.ArticlePubMed
- 41. Barber HO, Dionne J. Vestibular findings in vertebro-basilar ischemia. Ann Otol Rhinol Laryngol 1971;80:805–12.ArticlePubMedPDF
- 42. Yang CS, Young YH. Clinical investigation on hypotensive patients with vertigo. Eur Arch Otorhinolaryngol 2006;263:804–8.ArticlePubMedPDF
- 43. Kim HA, Bisdorff A, Bronstein AM, Lempert T, RossiIzquierdo M, Staab JP, et al. Hemodynamic orthostatic dizziness/vertigo: diagnostic criteria. J Vestib Res 2019;29:45–56.ArticlePubMedPMC
- 44. Kim HA, Yi HA, Lee H. Recent advances in orthostatic hypotension presenting orthostatic dizziness or vertigo. Neurol Sci 2015;36:1995–2002.ArticlePubMedPDF
- 45. Jeon EJ, Park YS, Park SN, Park KH, Kim DH, Nam IC, et al. Clinical significance of orthostatic dizziness in the diagnosis of benign paroxysmal positional vertigo and orthostatic intolerance. Am J Otolaryngol 2013;34:471–6.ArticlePubMed
- 46. Parfenov VA, Ostroumova TM, Ostroumova OD. Hypertension and dizziness: is there a relation? Ration Pharmacother Cardiol 2019;15:125–9.ArticlePDF
- 47. Ito F, Tanaka K, Kamada H. Vertigo secondary to hypotension: the relationship between therapeutic effects and hearing. Auris Nasus Larynx 1998;25:161–7.ArticlePubMed
- 48. Pirodda A, Ferri GG, Montana T, Riggio R, Innocenti G, Di Nino G. Hypotension as an isolated factor may not be sufficient to provoke hearing impairment. J Laryngol Otol 2004;118:941–5.ArticlePubMed
- 49. Kawakami M, Makimoto K, Fukuse S, Takahashi H. Autoregulation of cochlear blood flow. A comparison of cerebral blood flow with muscular blood flow. Eur Arch Otorhinolaryngol 1991;248:471–4.ArticlePubMedPDF
- 50. Shulman A, Goldstein B. Brain and inner-ear fluid homeostasis, cochleovestibular-type tinnitus, and secondary endolymphatic hydrops. Int Tinnitus J 2006;12:75–81.PubMed
- 51. Matsumura Y, Y amanaka T, Murai T, Fujita N, Kitahara T. Orthostatic hemodynamics in the vertebral artery and blood pressure in patients with orthostatic dizziness/vertigo. Auris Nasus Larynx 2022;49:593–8.ArticlePubMed
- 52. Mayo Clinic. Heart valve disease: symptoms and causes [Internet]. Rochester (MN), Mayo Clinic. c1998-2022;[cited 2022 Feb 2]. Available from: https://www.mayoclinic.org/diseases-conditions/heart-valve-disease/symptoms-causes/syc-20353727.
- 53. Pick A. Adam’s Blog: “Dizzy after heart valve surgery?” Asks Rick. [Internet]. Torrance (CA), HeartValveSurgery.com. 2009;[cited 2022 Feb 2]. Available from: https://www.heart-valvesurgery.com/heart-surgery-blog/2009/09/09/dizzy-after-heartsurgery/.
- 54. Susanto M. Dizziness: if not vertigo could it be cardiac disease? Aust Fam Physician 2014;43:264–9.PubMed
- 55. Brignole M. Diagnosis and treatment of syncope. Heart 2007;93:130–6.ArticlePubMedPMC
- 56. Shoair OA, Nyandege AN, Slattum PW. Medication-related dizziness in the older adult. Otolaryngol Clin North Am 2011;44:455–71.ArticlePubMed
- 57. Juraschek SP, Simpson LM, Davis BR, Beach JL, Ishak A, Mukamal KJ. Effects of antihypertensive class on falls, syncope, and orthostatic hypotension in older adults: the ALLHAT trial. Hypertension 2019;74:1033–40.ArticlePubMedPMC
- 58. Koskenoja M, Orma EJ. Postural dizziness in the aged. Geriatrics 1957;12:49–59.PubMed
- 59. Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA 1989;261:2663–8.ArticlePubMed
- 60. Ensrud KE, Nevitt MC, Yunis C, Hulley SB, Grimm RH, Cummings SR. Postural hypotension and postural dizziness in elderly women. The study of osteoporotic fractures. The Study of Osteoporotic Fractures Research Group. Arch Intern Med 1992;152:1058–64.ArticlePubMed
- 61. Pirodda A, Brandolini C, Ferri GG, Modugno GC, Esposti DD, Borghi C. Inner ear dysfunction of uncertain origin: a multidisciplinary approach could give something more. Med Hypotheses 2009;72:188–9.ArticlePubMed
- 62. Myers MG, Kearns PM, Kennedy DS, Fisher RH. Postural hypotension and diuretic therapy in the elderly. Can Med Assoc J 1978;119:581–5.PubMedPMC
- 63. McDowell FH, Lee JE. Levodopa, Parkinson's disease, and hypotension. Ann Intern Med 1970;72:751–2.ArticlePubMed
- 64. Lezama-Martínez D, Valencia-Hernández I, Flores-Monroy J, Martínez-A guilar L. Combination of β adrenergic receptor block and renin-angiotensin system inhibition diminished the angiotensin II-induced vasoconstriction and increased bradykinin-induced vasodilation in hypertension. Dose Response 2017;15:1559325817737932. PubMedPMC
- 65. Chimirri S, Aiello R, Mazzitello C, Mumoli L, Palleria C, Altomonte M, et al. Vertigo/dizziness as a drugs' adverse reaction. J Pharmacol Pharmacother 2013;4(Suppl 1):S104–9.ArticlePubMedPMCPDF
- 66. Faruqui AA. Evaluation of safety and efficacy of telmisartan-amlodipine combination in treating hypertension. J Indian Med Assoc 2008;106:612. –4. 624.PubMed
- 67. Leon MB, Rosing DR, Bonow RO, Epstein SE. Combination therapy with calcium-channel blockers and beta blockers for chronic stable angina pectoris. Am J Cardiol 1985;55:69B–80B.ArticlePubMed
- 68. Strauss WE, Parisi AF. Combined use of calcium-channel and beta-adrenergic blockers for the treatment of chronic stable angina. Rationale, efficacy, and adverse effects. Ann Intern Med 1988;109:570–81.ArticlePubMed
- 69. Krikler DM, Harris L, Rowland E. Calcium-channel blockers and beta blockers: advantages and disadvantages of combination therapy in chronic stable angina pectoris. Am Heart J 1982;104:702–8.ArticlePubMed
- 70. Argaet EC, Bradshaw AP, Welgampola MS. Benign positional vertigo, its diagnosis, treatment and mimics. Clin Neurophysiol Pract 2019;4:97–111.ArticlePubMedPMC
- 71. Halmagyi GM. Diagnosis and management of vertigo. Clin Med (Lond) 2005;5:159–65.ArticlePubMedPMC
Citations
Citations to this article as recorded by




 KBS
KBS

 PubReader
PubReader ePub Link
ePub Link Cite
Cite