급성 현훈으로 발현한 신생물딸림소뇌변성
Paraneoplastic Cerebellar Degeneration Presented as Acute Vertigo
Article information
Trans Abstract
Paraneoplastic cerebellar degeneration is a rare neurological manifestation of nonmetastatic malignancy. Its usual manifestation is subacute to chronic dizziness, gait ataxia, and dysarthria. There have been only a few cases of paraneoplastic cerebellar degeneration with acute presentation. This study describes a patient with paraneoplastic cerebellar degeneration, who presented acute vestibular syndrome and then episodically developed horizontal gaze-evoked nystagmus and gait ataxia.
INTRODUCTION
Paraneoplastic cerebellar degeneration refers to a rare neurological manifestation of nonmetastatic malignancy. Its usual manifestation is subacute to chronic dizziness, gait ataxia, and dysarthria. There have been only a few cases of paraneoplastic cerebellar degeneration with acute presentation in the literature. This study aims to describe a patient with paraneoplastic cerebellar degeneration, who initially presented as acute vertigo mimicking unilateral vestibulopathy.
CASE REPORT
A 44-year-old woman with hyperlipidemia developed sudden vertigo and headache. Two years before she was diagnosed with ovarian cancer, and had bilateral salipoophorectomy and total abdominal hysterectomy along with adjuvant chemotherapy. Examination on the 4th day from the vertigo onset found small horizontal nystagmus beating to left-down-counterclockwise direction in darkness (Fig. 1A), which increased just after horizontal head shaking. There was no gaze-evoked nystagmus. Horizontal saccades and smooth pursuit were normal. She showed leftward veering tendency during enhanced Romberg test. There was no other neurologic deficit. The video head impulse tests revealed preserved vestibulo-ocular reflex gain but with small catch-up saccades for right horizontal canal (Fig. 1B). Bithermal caloric tests showed borderline canal paresis of the right ear and high directional preponderance to leftward. Brain magnetic resonance imaging (MRI) was unremarkable. Her symptoms improved over the next 4 days, and she reported near resolution of vertigo and headache. However, examination performed on the 12th day from the symptom onset found small rightbeat spontaneous nystagmus and gaze-evoked nystagmus during eccentric lateral gaze. Follow-up diffusion-weighted image was normal. Video-oculography showed right-down-clockwise beating spontaneous nystagmus without fixation and rightbeat head shaking nystagmus. Video head impulse tests showed decreased gains with catch-up saccades for both posterior canals. Pure tone audiometry was normal. Examination on the 19th day from the symptom onset showed normal findings, only except rightward veering upon enhanced Romberg.
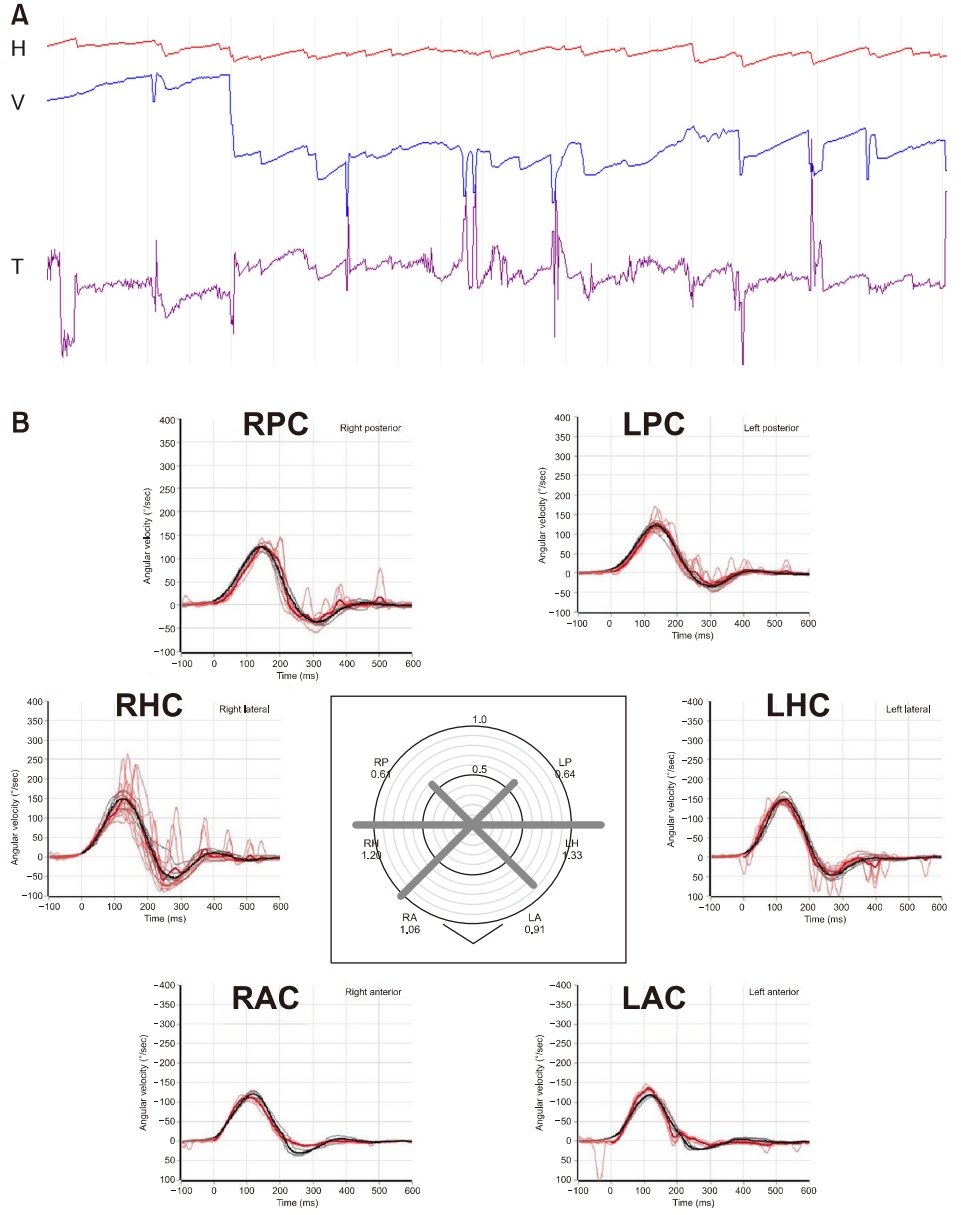
(A) Examination on the 4th day from the vertigo onset found small horizontal nystagmus beating toward left-down-counterclockwise direction in darkness. (B) Recording of the head impulse tests showed normal range of vestibulo-ocular reflex gain but with covert catch-up saccades for right horizontal canal (B). H, horizontal position of the eye; V, vertical position of the eye; T, torsional position of the eye; upward deflection indicates rightward, upward, and clockwise eye motion; RAC, right anterior canal; LAC, left anterior canal; RHC, right horizontal canal; LHC, left horizontal canal; RPC, right posterior canal; LPC, left posterior canal.
After the next 2 months, she experienced recurred dizziness. Examination found no spontaneous nystagmus with and without fixation, but small left-downbeat nystagmus during leftward gaze. Convergence induced rightbeat nystagmus. Horizontal head shaking produced right-downbeat nystagmus. Horizontal saccades and smooth pursuit were normal, while upward saccades were slightly hypermetric (Fig. 2A). Video head impulse tests recorded slightly early acceleration for right horizontal canal (Fig. 2B). She eventually developed wide-based ataxic gait. During the next 2 months, she showed distinct horizontal gaze-evoked nystagmus, dysarthria, and marked gait ataxia. Follow-up brain MRI was unremarkable. Cerebrospinal fluid analysis did not show malignant cells. Serum paraneoplastic panel detected anti-neuronal (anti-Yo) antibody. A trial of intravenous imunoglobulin failed to produce the improvement of the syndrome. Six months after the onset of the symptoms the patient was unable to walk.

Examination 4 months later from the vertigo onset showed intermittent hypermetric upward saccades and pulse-step mismatch during vertical saccades (A). The video head impulse tests showed slightly early acceleration and increased peak for right anterior canal (B). V, vertical position of the eye; upward deflection indicates upward eye motion; RAC, right anterior canal; LAC, left anterior canal; RHC, right horizontal canal; LHC, left horizontal canal; RPC, right posterior canal; LPC, left posterior canal.
DISCUSSION
The acute manifestation of vertigo and imbalance in our patient made more likely a diagnosis of acute vestibular syndrome rather than degenerative or neoplastic etiologies. The vestibular asymmetry deduced from unilaterality of horizontal-torsional spontaneous and head shaking nystagmus and unilateral truncal veering upon enhanced Romberg test, as well as a following transient recovery period, also suggested the possibility of acute vestibular syndrome. However, follow-up examinations found the reversal of spontaneous nystagmus, fluctuating presentation of gaze-evoked nystagmus, and gradual development of gait ataxia, directing to the investigation toward the paraneoplastic cerebellar degeneration. Paraneoplastic cerebellar degeneration usually has a subacute or chronic manifestation progressive over several weeks and months [1]. There have been only few cases of paraneoplastic cerebellar degeneration with acute manifestation associated with small cell lung cancer, ovarian carcinoma, malignant neuroendocrine tumor of the stomach, and Hodgkin’s lymphoma [2,3].
Immunological responses to neuronal antigens expressed by the underlying cancer are supposed to be a pathogenesis of the paraneoplastic disorders. Anti-Yo antibody is an anti-Purkinje cell cytoplasmic antibody found in approximately 50% of patients with paraneoplastic cerebellar degeneration and has been reported exclusively in breast or gynecological malignancies [4]. Eye movement abnormalities are various, including downbeat nystagmus, opsoclonus, skew deviation, slow or inaccurate saccades, impaired smooth pursuit, and gaze-evoked nystagmus [5]. While those ocular motor manifestations indicate from diffuse cerebellar Purkinje cell loss with proliferation of Bergmann glia and sometimes infiltration of inflammatory cells in the deep cerebellar nuclei [3], the initial vestibular asymmetry found in the current case may imply asymmetric initiation of the pathologic cellular loss or infiltrative changes in paraneoplastic cerebellar degeneration.
Paraneoplastic neurological disorders are much less common than direct, metastatic, and treatment-related complications of cancer, but are nevertheless important because they cause severe neurological morbidity and mortality and frequently present to the neurologist in a patient without a known malignancy [6]. In more than half of the cases of paraneoplastic cerebellar degeneration the syndrome emerges before the clinical appearance of cancer [1,7]. In cases of a vestibular syndrome of acute manifestation, where the initial investigation excludes the most common causes such as vascular events or inflammatory disease, the differential diagnosis requires to include paraneoplastic cerebellar degeneration.
Notes
No potential conflict of interest relevant to this article was reported.
