Abstract
-
Objectives
- Endolymphatic sac decompression surgery (ESDS) is one of the surgical methods for intractable Menière’s disease (MD), and it is known as a relatively safe treatment that does not cause hearing loss. However, the effectiveness and the degree of vertigo control rate of ESDS are still controversial. In this study, we aimed to evaluate the clinical outcomes of ESDS in intractable MD.
-
Methods
- We retrospectively reviewed 33 patients who underwent ESDS for intractable MD from January 2002 to March 2022. Clinical characteristics of patients, pure tone threshold, medical treatment method, and number of vertigo attacks before and after surgery were assessed. The improvement of hearing and vertigo was evaluated according to the 1995 American Academy of Otolaryngology-Head and Neck Surgery criteria.
-
Results
- Of the 33 patients, the average follow-up period was 21.2 months, with immediate follow-up within 2 months (28 patients), short term between 2 and 6 months (27 patients), and long term at 12 months or later (29 patients). In the immediate hearing threshold, both air conduction and bone conduction showed slight deterioration, but there was no significant change in the long-term hearing threshold. At long-term follow-up, 12 patients (41.4%) were able to live without medication, and 18 patients (62.1%) showed improvement in their vertigo symptoms. In addition, patients who showed improvement in hearing also showed improvement in vertigo at the same time.
-
Conclusions
- ESDS in intractable MD is a relatively safe and effective treatment method for reducing vertigo attack without worsening hearing threshold.
-
Keywords: Meniere disease; Endolymphatic sac; Surgical decompression; Vertigo; Endolymphatic hydrops
-
중심단어: 메니에르병, 내림프낭, 감압술, 현훈, 내림프수종
INTRODUCTION
Menière’s disease (MD) is a condition characterized by recurrent episodes of vertigo, fluctuating hearing loss, tinnitus, and aural fullness. It is believed to be associated with endolymphatic hydrops (EH), an abnormal accumulation of fluid in the inner ear. The pathogenesis of MD is not fully understood, although several factors such as vascular abnormalities, viral infection, ionic imbalance, autoimmune factors, and genetic predisposition have been proposed as contributing factors [1].
The management of MD typically begins with conservative approaches, including lifestyle modifications, dietary changes, and medication to alleviate symptoms and reduce the frequency of vertigo attacks. However, in cases where conservative measures fail to provide sufficient relief, more invasive treatments such as intratympanic gentamicin injection or endolymphatic sac decompression surgery (ESDS) may be considered [1,2].
ESDS is a surgical intervention that aims to alleviate symptoms and improve the quality of life for patients with intractable MD [3]. It is a minimally destructive procedure that involves the creation of a mastoid shunt to decompress the endolymphatic sac, thereby reducing fluid pressure within the inner ear [4]. ESDS has been reported to provide effective vertigo control and improve hearing outcomes in certain patients, particularly those with preserved functional hearing [5]. However, the efficacy of ESDS is still controversial, with one study [3] reporting improvement in symptoms in 95% of patients, while another study [6] found no significant difference between the group that received ventilation tube insertion alone and the group that underwent surgery. The difficulty in determining the effectiveness of ESDS may be attributed to the challenges of completely blinding the placebo effect associated with surgery and the limited number of surgical procedures performed. Therefore, in this study, we aimed to evaluate the clinical outcomes of ESDS in patients with intractable MD who underwent the procedure at our institution.
MATERIALS AND METHODS
This investigation was approved by the Institutional Review Board of Samsung Medical Center (No. 2022-09-057) and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all the patients.
1. Patients and Study Design
A retrospective review of medical records was conducted for patients who underwent ESDS for intractable MD from January 2002 to March 2022. The inclusion criteria were based on the diagnostic criteria for definite MD as defined by the Bárány Society in 2015 [7]. Patients were defined as having intractable MD when they presented with one or more recurrent vertigo attacks to compromise daily life with fluctuating or progressive hearing loss despite systematic medical treatment including lifestyle modification and pharmacological therapy (including diuretics, betahistine, and isosorbide) for at least 3 months and were considered for ESDS [8]. Finally, a total of 33 patients were included in the study. The patients’ baseline characteristics, treatment methods, pre- and postoperative pure tone thresholds, frequency of vertigo attacks, and follow-up periods were assessed by reviewing medical records. The follow-up period included immediate (within 2 months postsurgery), short-term (between 2 and 6 months postsurgery), and long-term (12 months and beyond postsurgery) assessments, which corresponded to data collected at 1 month, 6 months, and 2 years after surgery, respectively. Due to the retrospective nature of the study, the duration of follow-up varied among patients; and therefore, data collection was limited to the closest available information within the specified time ranges.
2. Surgical Procedure
The ESDS procedure was performed under general anesthesia by a single surgeon. A postauricular incision was made a few millimeters posterior to the postauricular sulcus. Subsequently, a simple mastoidectomy was conducted, involving the removal of bone in the mastoid region to access the middle and posterior cranial fossa. During this step, a meticulous examination of structures such as the incus, lateral semicircular canal, and posterior semicircular canal was carried out. The sigmoid sinus was then carefully skeletonized, followed by further drilling along the posterior fossa dura. In accordance with Donaldson’s line, an imaginary line extending from the lateral semicircular canal and bisecting the posterior semicircular canal, the inferior aspect of the endolymphatic sac was identified. An incision was subsequently made in the endolymphatic sac, and a silastic sheet was inserted. Finally, after the application of glue, the surgical procedure was concluded.
3. Assessment of Outcomes
All patients underwent pure tone audiometry before and after surgery, with average values at 0.5, 1, 2, and 4 kHz used as the hearing thresholds. The assessment of hearing outcomes was categorized into three groups according to the 1995 American Academy of Otolaryngology-Head and Neck Surgery (AAOHNS) criteria [9]: improved group (hearing gain, ≥10 dB), stable group (hearing gain, ≥‒10 and <10 dB), and worsened group (hearing gain, <‒10 dB). The evaluation of symptoms also followed the 1995 AAO-HNS criteria [9], with numerical values assigned and used to categorize patients into three groups. Patients with a significant decrease in the frequency of vertigo attacks after surgery, with a score between 1% and 40% of the preoperative monthly average, were classified as the ‘improved’ group. Patients with no significant change in postoperative vertigo attack frequency and scores between 41 and 120% of the preoperative frequency were in the ‘stationary’ group. The ‘worsened’ group was classified as patients whose postoperative frequency increased to more than 120% of the initial monthly average or who required secondary treatment due to insufficient control of vertigo (Table 1). Changes in treatment methods were determined by comparing the number of medications used before and after surgery, and classified as follows: add medication (additional medication or secondary treatment initiated due to vertigo), keep medication, reduce medication, and no medication (discontinuation of medication).
4. Statistical Analysis
Statistical analysis was conducted using IBM SPSS Statistics ver. 26.0 (IBM Corp.). Continuous variables were presented as means±standard deviations, while categorical variables were described using frequencies and percentages. The paired t-test was used to compare the changes in hearing and the frequency of vertigo attacks before and after treatment. In addition, the Fisher exact test and one-way analysis of variance were used to assess the differences in vertigo outcomes among different hearing groups. A two-tailed p-value of <0.05 was considered statistically significant.
RESULTS
1. Patient Characteristics
Among the total of 33 patients who underwent ESDS, 19 (57.6%) were male and 14 (42.4%) were female. The mean age of the patients was 48.1±16.0 years. The affected side was predominantly the right side in 21 patients (63.6%) and the left side in 12 patients (36.4%). The initial hearing threshold of the overall patient population was 46.9±17.5 dB, and no air-bone gap (ABG) was observed. Prior to surgery, the average number of vertigo attacks experienced by the patients was 5.4 per month, and the duration of the disease was 48.2±53.3 months. The total follow-up period was 21.2±10.8 months. During the follow-up period, no patient developed symptoms in the contralateral ear, and no postoperative complications were observed in any of the patients (Table 2).
2. Audiological Outcomes
A total of 28 patients were included in the immediate follow-up group, 27 patients in the short-term follow-up group, and 29 patients in the long-term follow-up group, respectively (Table 3). Fig. 1 shows the individual changes in hearing threshold. According to the AAO-HNS criteria, in the long term, five patients (17.2%) were classified as the improved group, 12 patients (41.4%) in the stationary group, and 12 patients (41.4%) in the worsened group. Analyzing the average hearing thresholds across the different time periods, the immediate period showed a significant deterioration in both air conduction and bone conduction compared to preoperative values. On the other hand, the short-term hearing demonstrated a slight increase in air conduction and a slight decrease in bone conduction, resulting in a significant increase in the ABG compared to preoperative measurements. However, no significant differences were observed in air conduction, bone conduction, or ABG between preoperative and postoperative measurements in the long term (Table 4).
3. Treatment Methods
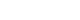
Prior to surgery, all patients received pharmacological treatment. All patients received diuretics, of which 20 patients (60.6%) used betahistine with diuretics and four patients (12.1%) used isosorbide with diuretics. Immediately after surgery, the majority of patients (19, 67.9%) maintained their preoperative medication regimen. Five patients (17.9%) reduced their medication, and four patients (14.3%) experienced symptom control without the need for medication. In the short term, most patients either maintained or reduced their medication, except for one patient (3.6%) who required additional medication. In the long term, 12 patients (41.4%) were able to manage their daily lives without medication. However, among the six patients (20.7%) who did not achieve symptom control, five patients (17.2%) received additional treatment with intratympanic gentamicin injection, and one patient (3.4%) underwent labyrinthectomy (Fig. 2).
4. Symptom Control
During the follow-up period, the frequency of vertigo attacks showed a significant decrease at all time points compared to pre-surgery levels (Fig. 3). When classified according to the vertigo class mentioned earlier, immediate improvement in symptoms was observed in 18 patients (64.3%), short-term improvement in 22 patients (78.6%), and long-term improvement in 18 patients (62.1%) (Fig. 4).
5. Relationship between Hearing Outcome and Symptom Control
At the short-term time point, which exhibited the most favorable outcomes following surgery, we analyzed the correlation between hearing outcome and symptom control. In terms of the average vertigo frequency before surgery, there was no significant difference among the hearing recovery groups (p=0.615). However, postsurgery, we observed a significant difference in the average number of vertigos between the improved and worsened groups (p=0.033). Furthermore, when considering the vertigo class, the improved group showed complete symptom control, while only 63.6% of the worsened group demonstrated symptom improvement, but there was no significant difference between the groups (p=0.552) (Table 5).
DISCUSSION
In this study, we evaluated the clinical outcomes of ESDS in patients with intractable MD. Our results showed a significant reduction in the frequency of vertigo attacks after surgery and a relatively stable hearing threshold in the long term. Notably, the short-term outcomes were particularly promising, with approximately 60% of patients not experiencing hearing deterioration and 78% showing improvement in vertigo symptoms. In the long term, 41% of patients were able to live without medication. Additionally, patients who showed improvement in hearing also showed improvement in vertigo at the same time.
The pathophysiology of MD is believed to be related to EH. In the past, the presence of EH in patients with MD could only be confirmed through postmortem histopathological examinations. However, recent advances in magnetic resonance imaging (MRI) techniques have enabled the visualization of EH in patients with MD through radiological examination. In 2010, Naganawa et al. [10] first established a technique for diagnosing EH using intravenous gadolinium-enhanced inner ear MRI, making it possible to visualize EH. In 2018, Cho et al. [11] utilized this technique and reported that the degree of EH in MD was significantly higher compared to the unaffected side, and a significant correlation was found between the degree of EH and audiological outcomes.
The endolymphatic sac, located on the posterior surface of the petrous portion of the temporal bone and connected to the vestibule via the endolymphatic duct, plays a key role in the regulation of volume and pressure of the endolymph in the inner ear organ [12]. ESDS involves creating a shunt in the endolymphatic sac, reducing the pressure of the endolymph and thereby helping to restore peripheral vestibular function and improve vertigo [4,13]. Unlike intratympanic gentamicin injection, a type of chemical labyrinthectomy, ESDS has the advantage of preserving hearing.
1. Audiological Outcomes
The hearing preservation rate after ESDS has been reported with a broad range between 53% and 94% [4,5,14,15]. In this study, the short-term hearing preservation rate was 59.2%, and the long-term hearing preservation rate was 58.6%. These rates are in line with the previously reported figures, although they are somewhat lower. However, when examining the average hearing levels, both short-term and long-term hearing thresholds did not show a significant difference compared to preoperative hearing thresholds.
An immediate postoperative assessment showed that 60.7% of patients experienced deterioration in their hearing, with an average hearing threshold significantly elevated. Given the subsequent improvement in hearing, this is thought to be due to temporary surgical trauma. During the short-term follow-up, we observed a temporary increase in the ABG. This is thought to have resulted from a temporary third window effect due to the procedure’s nature where a shunt is created using a silastic sheet, rather than merely achieving decompression.
Nevertheless, it is unclear whether the hearing deterioration seen in 40.7% of patients was influenced by this effect, and it is challenging to explain the gradual deterioration of hearing observed in long-term follow-up. Another hypothesis suggests that the hearing deterioration in long-term follow-ups could be due to the natural progression of MD, independent of surgery. This may imply that while ESDS may aid in providing temporary improvements, it may not be able to prevent the long-term progression of the disease.
2. Symptom Control and Changes in Treatment Strategies
Generally, the success rate of vertigo control with ESDS is reported to be as high as 70-80% in most literature [4,5,16-18]. This study also showed significant improvement in symptoms immediately after the operation, in contrast to the temporary decrease in hearing. At the immediate follow-up, 64.3% showed improvement in symptoms, and the symptom improvement rate increased up to 78.6% at the short-term follow-up. In the long term, 62.1% showed improvement in vertigo as six patients received additional treatments. The average number of vertigo episodes per month also showed a significant decrease immediately after surgery. Immediately after the operation, most patients maintained their medication even if their symptoms improved (67.9%), and the number of patients who could maintain their symptoms without medication gradually increased over the follow-up period, with 41% being able to live without medication in the long term.
However, while the number of postoperative vertigo attacks decreased and the number of patients who did not require medication increased, the number of patients whose symptoms worsened over time also increased. In total, six patients received additional treatments due to the persistence or worsening of symptoms, which led to a lower success rate during long-term follow-up. They were classified as the ‘worsened group’ by vertigo class and the ‘additional medication’ group by treatment method. All of these six patients belonged to the symptomimproved (four patients) or stationary group (two patients) at the short-term follow-up. However, they experienced symptom aggravation after 6 months postoperation, which led to additional treatment. Considering this, rather than the surgery itself bringing about long-term symptom worsening, it is thought that the symptoms worsened due to the course of MD, which aligns with the context explained earlier.
3. Natural Course Following Treatment for Menière’s Disease
In previous studies, it has been reported that between 7% and 37% of patients require additional treatment due to persistent symptoms after ESDS. This is consistent with our result of 18.2% (6 out of 33). While a clear cause cannot be identified for this, one study reported that upon reexploring the previous surgical site through revision surgery, the surgical site was found to be blocked due to granulation tissue around the shunt [18]. This suggests that even when decompression is attempted through surgery, there is a possibility that the shunt site may be blocked again in some patients, and these patients could experience symptom worsening due to the typical course of MD. Therefore, when deciding to undergo ESDS, physicians should always consider the possibility that symptoms may worsen again in the long term and require further intervention.
There is a variety of reported rates of symptom improvement in MD patients who only receive conservative treatment without surgery. Tokumasu [19] reported that only 24% of 54 patients receiving conservative treatment for MD showed spontaneous symptom remission after being observed for 5 to 10 years. Moreover, Telischi and Luxford [18] reported a 42% remission rate during a 5-year follow-up among MD patients who did not undergo surgery. Filipo and Barbara [20] compared the progress of 20 patients who received ESDS and 20 patients who refused surgery despite having the same indications for surgery. In the surgical group, 50% experienced symptom improvement, while only 30% of the nonsurgical group showed symptom improvement. Thus, even considering the possibility of symptom recurrence, the long-term vertigo improvement rate of 62.1% is higher than the symptom improvement rate after conservative treatment, reinforcing the usefulness of ESDS.
4. Relationship between Hearing Outcome and Symptom Control
Our study shows a correlation between hearing outcome and the degree of symptom control. In the hearing-worsened group, the number of vertigo occurrences postsurgery was significantly higher than in the hearing-improved group, with all patients in the hearing-improved group showing an improvement in vertigo. A previous study has shown a significant positive correlation between the EH ratio and hearing threshold and also revealed a relationship with electrocochleography (ECoG), cervical vestibular-evoked myogenic potentials, and caloric test results. Considering the hypothesis that hearing loss and vertigo attacks in MD occur due to EH, our results align with those of previous studies showing a correlation between the two. However, our study was limited by the fact that we were unable to observe the degree of EH, necessitating further research on whether the actual degree of EH decreases after surgery.
This study has several limitations. First, the study was designed retrospectively and involved a small number of patients with an inconsistent follow-up period. Second, there was no control group in this study, which limits the ability to objectively compare the treatment outcomes. Future studies with gender- and age-matched comparisons of patients with long-term follow-up without ESDS are needed. Third, we were unable to comprehensively compare results from other tests such as vestibular function tests, ECoG, etc. Fourth, like previous studies, it was an inability to rule out the placebo effect caused by surgery. Finally, we did not measure EH in patients before and after surgery. Hence, there is a need for further prospective research.
Despite these limitations, this study carries the strength of providing long-term follow-up results for the therapeutic effects of ESDS. In conclusion, ESDS in intractable MD presents as a beneficial approach that offers reliable vertigo control and supports auditory stability. However, it is important to consider the possibility that vertigo may worsen again during long-term follow-up in some patients.
ARTICLE INFORMATION
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING/SUPPORT
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization, Supervision: WHC; Data curation, Formal analysis: HWS; Investigation, Methodology: YSC; Project administration, Validation: YSC, WHC; Visualization: HSW, WHC; Writing–Original Draft: HWS; Writing–Review & Editing: All authors.
All authors read and approved the final manuscript.
Fig. 1.Changes of pure tone audiometry threshold (air conduction) during the follow-up period.

Fig. 2.Changes in medical treatment methods during the follow-up period.
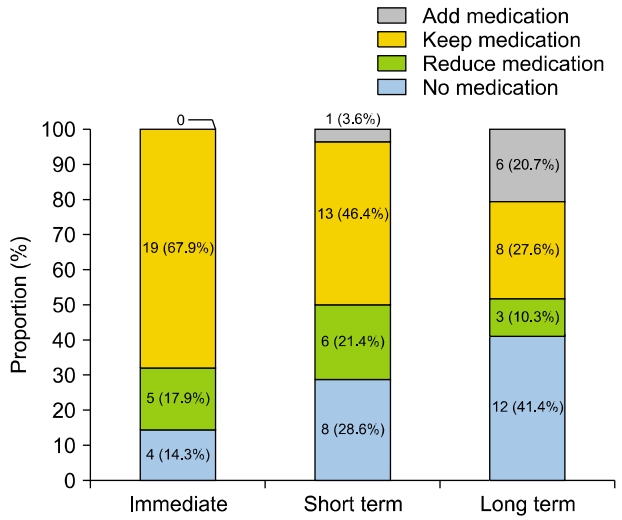
Fig. 3.Number of vertigo attacks (per month) over the follow-up period.
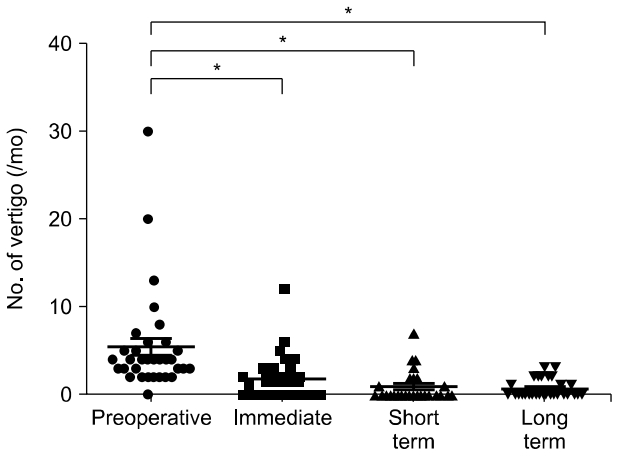
Fig. 4.Vertigo control rate during the follow-up period.
Table 1.Categorization of symptom control levels into vertigo classes
|
Vertigo class |
Numerical value |
|
Improved |
1–40 |
|
Stationary |
41–120 |
|
Worsened |
>120 or Secondary treatment initiated due to vertigo |
Table 2.Demographic and clinical characteristics of the study population
|
Variable |
Data |
|
No. of patients |
33 |
|
Sex, male:female |
19 (57.6):14 (42.4) |
|
Age (yr) |
48.1±16.0 |
|
Affected side, right:left |
21 (63.6):12 (36.4) |
|
Initial hearing threshold (dB) |
|
|
Air conduction |
46.9±17.5 |
|
Bone conduction |
42.8±16.2 |
|
Air-bone gap |
4.4±3.9 |
|
No. of vertigo attack before surgery (/mo) |
5.4±5.8 |
|
Dizziness duration (mo) |
48.2±53.3 |
|
Follow-up period (mo) |
21.2±10.8 (6.3–58.8) |
|
Postoperative complications |
0 (0) |
Table 3.Hearing outcomes during follow-up period
|
Outcome |
Immediate (n=28) |
Short term (n=27) |
Long term (n=29) |
|
Improved |
0 (0) |
5 (18.5) |
5 (17.2) |
|
Stationary |
11 (39.3) |
11 (40.7) |
12 (41.4) |
|
W orsened |
17 (60.7) |
11 (40.7) |
12 (41.4) |
Table 4.Average of pure tone audiometry threshold over the follow-up period
|
Variable |
Preoperative (n=33) |
Immediate (n=28) |
Short term (n=27) |
Long term (n=29) |
|
Pure tone threshold |
|
|
|
|
|
Air conduction (dB) |
46.9±17.5 |
59.0±21.5*
|
47.9±19.0 |
55.5±23.1 |
|
Bone conduction (dB) |
42.8±16.2 |
47.4±17.4*
|
38.6±16.9 |
47.8±16.3 |
|
Air-bone gap (dB) |
4.4±3.9 |
11.6±7.7*
|
9.4±6.8*
|
8.0±9.9 |
|
Follow-up period (mo) |
|
1.0±0.5 (0.2–2.0) |
4.8±0.8 (3.3–6.0) |
23.1±10.1 (12.0–58.8) |
Table 5.Degree of vertigo control according to hearing outcomes at short-term follow-up
|
Variable |
Hearing outcomes
|
p-value |
|
Improveda) (n=5) |
Stationaryb) (n=1) |
Worsenedc) (n=11) |
|
No. of vertigo (/mo) |
|
|
|
|
|
Preoperative |
3.8±1.5 |
3.8±3.3 |
4.9±2.6 |
0.615 |
|
Short term |
0 |
0.5±0.8 |
2.0±2.3*
|
0.033 |
|
Vertigo class |
|
|
|
0.552 |
|
Improved |
5 (100) |
9 (81.8) |
7 (63.6) |
|
|
Stationary |
0 (0) |
2 (18.2) |
2 (18.2) |
|
|
Worsened |
0 (0) |
0 (0) |
2 (18.2) |
|
REFERENCES
- 1. Basura GJ, Adams ME, Monfared A, Schw artz SR, Antonelli PJ, Burkard R, et al. Clinical practice guideline: Ménière’s disease. Otolaryngol Head Neck Surg 2020;162(2 Suppl):S1–55.
- 2. Nevoux J, Barbara M, Dornhoffer J, Gibson W, Kitahara T, Darrouzet V. International consensus (ICON) on treatment of Ménière’s disease. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135(1 Suppl):S29–32.ArticlePubMed
- 3. Durland WF Jr, Pyle GM, Connor NP. Endolymphatic sac decompression as a treatment for Meniere’s disease. Laryngoscope 2005;115:1454–7.ArticlePubMed
- 4. Suh MW, Kim BJ, Kim CS. Long term treatment results of endolymphatic sac decompression in Meniere’s disease. Korean J Otorhinolaryngol-Head Neck Surg 2008;51:319–25.
- 5. Ostrowski VB, Kartush JM. Endolymphatic sac-vein decompression for intractable Meniere’s disease: long term treatment results. Otolaryngol Head Neck Surg 2003;128:550–9.ArticlePubMedPDF
- 6. Hellström S, Thomsen J, et al. The non-specific effect of endolymphatic sac surgery in treatment of Meniere’s disease: a prospective, randomized controlled study comparing “classic” endolymphatic sac surgery with the insertion of a ventilating tube in tympanic membrane. Acta Oto-Laryngol 1998: 118:769-773. Acta Otolaryngol 2018;138:304–9.PubMed
- 7. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Menière’s disease: consensus document of the Bárány Society, the Japan Society for Equilibrium Research, the European Academy of Otology and Neurotology (EAONO), the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) and the Korean Balance Society. Acta Otorrinolaringol Esp 2016;67:1–7.PubMed
- 8. Kitahara T. Evidence of surgical treatments for intractable Meniere’s disease. Auris Nasus Larynx 2018;45:393–8.ArticlePubMed
- 9. Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière’s disease. Otolaryngol Head Neck Surg 1995;113:181–5.PubMed
- 10. Naganawa S, Yamazaki M, Kawai H, Bokura K, Sone M, Nakashima T. Visualization of endolymphatic hydrops in Ménière’s disease with single-dose intravenous gadolinium-based contrast media using heavily T(2)-weighted 3D-FLAIR. Magn Reson Med Sci 2010;9:237–42.ArticlePubMed
- 11. Cho YS, Ahn JM, Choi JE, Park HW, Kim YK, Kim HJ, et al. Usefulness of intravenous gadolinium inner ear MR imaging in diagnosis of Ménière’s disease. Sci Rep 2018;8:17562. ArticlePubMedPMCPDF
- 12. Couloigner V, Teixeira M, Sterkers O, Rask-Andersen H, Ferrary E. The endolymphatic sac: its roles in the inner ear. Med Sci (Paris) 2004;20:304–10.ArticlePubMed
- 13. Shah DK, Kartush JM. Endolymphatic sac surgery in Meniere’s disease. Otolaryngol Clin North Am 1997;30:1061–74.ArticlePubMed
- 14. Goin DW, Mischke RE, Esses BA, Young D, Priest EA, Whitmoyer-Goin V. Hearing results from endolymphatic sac surgery. Am J Otol 1992;13:393–7.PubMed
- 15. Fukushima M, Kitahara T, Horii A, Inohara H. Effects of endolymphatic sac decompression surgery on endolymphatic hydrops. Acta Otolaryngol 2013;133:1292–6.ArticlePubMed
- 16. Smith WC, Pillsbury HC. Surgical treatment of Menière’s disease since Thomsen. Am J Otol 1988;9:39–43.PubMed
- 17. Zheng G, Liu Y, He J, Li S, Zhang Q, Duan M, et al. A comparison of local endolymphatic sac decompression, endolymphatic mastoid shunt, and wide endolymphatic sac decompression in the treatment of intractable Meniere’s disease: a short-term follow-up investigation. Front Neurol 2022;13:810352. ArticlePubMedPMC
- 18. Telischi FF, Luxford WM. Long-term efficacy of endolymphatic sac surgery for vertigo in Menière's disease. Otolaryngol Head Neck Surg 1993;109:83–7.PubMed
- 19. Tokumasu K, Fujino A, Yoshio S, Hoshino I. Prognosis of Menière’s disease by conservative treatment: retrospective study on the time course of the disease. Acta Otolaryngol Suppl 1995;519:216–8.PubMed
- 20. Filipo R, Barbara M. Natural course of Menière’s disease in surgically-selected patients. Ear Nose Throat J 1994;73:254–7.ArticlePubMedPDF
Citations
Citations to this article as recorded by





 KBS
KBS




 PubReader
PubReader ePub Link
ePub Link Cite
Cite