Abstract
- Vertebrobasilar dolichoectasia (VBD) is a rare vascular condition characterized by the elongation, widening, and tortuosity of the vertebrobasilar artery. It can lead to various symptoms due to compression of cranial nerves and brainstem structures. We report a rare case of VBD presenting as sudden sensorineural hearing loss (SSNHL) with vertigo and spontaneous downbeat nystagmus in a 65-year-old woman with a history of hypertension. Magnetic resonance imaging revealed a tortuous and dilated left vertebrobasilar artery compressing the brainstem and left 8th cranial nerve root entry zone, without signs of acute cerebellar stroke. The compression of the anterior inferior cerebellar artery and cervicomedullary junction was also noted, suggesting a vascular cause for her symptoms. Following high-dose steroid treatment, the patient showed significant hearing improvement. This case underscores the importance of considering VBD in patients with unexplained SSNHL and vertigo, highlighting the role of detailed vascular imaging in the diagnosis and management of such cases.
-
Keywords: Vertebrobasilar dolichoectasia; Sudden hearing loss; Sudden; Vertigo; Vestibulocochlear nerve
INTRODUCTION
Vertebrobasilar dolichoectasia (VBD) is characterized by elongation, widening, and tortuosity of the artery in the posterior brain circulation which is recognized as a rare condition with a prevalence of less than 0.05% in the general population [1]. The majority of cases of VBD are detected incidentally, although symptomatic patients may manifest as transient or cerebrovascular ischemic events, hemorrhage, and compressive signs and symptoms of the brainstem and cranial nerves [2,3]. Compression of the cranial nerves near the pons and midbrain can cause symptoms such as unilateral facial twitching, trigeminal neuralgia, hearing loss, and oculomotor palsy [4-7]. Approximately 40% of patients with VBD experience symptoms associated with the vestibulocochlear nerve, including tinnitus, hearing loss, and vertigo, which can be attributed to either a thrombotic ischemic vascular mechanism or a direct compressive mechanism [8]. Additionally, downbeat nystagmus can be triggered by compression of the brainstem in patients with VBD [4]. Therefore, it is reasonable to assess the possibility of VBD as an underlying cause of audiovestibular dysfunction.
Sudden sensorineural hearing loss (SSNHL) is usually defined by an abrupt onset of a decrease in hearing of 30 decibels (dB) or more over a minimum of three consecutive frequencies, occurring within a 72-hour period [9]. The underlying causes for SSNHL still remain unclear, but recent efforts have been made to investigate several vascular causes using magnetic resonance imaging (MRI) [10,11].
We present a case of a 65-year-old woman who experienced SSNHL accompanying vertigo with spontaneous downbeat nystagmus in which VBD was considered as the underlying cause, and would like to report the features of the case along with a review of the literature. This study was approved by the Institutional Review Board of Chosun Hospital in Gwangju, Korea (No. 2023-03-016). As this was a case study, the requirement for informed consent was waived.
CASE REPORT
A 65-year-old female patient complaining of sudden abrupt onset of left hearing loss, tinnitus, and spontaneous vertigo was referred to our hospital. The patient had a history of hypertension being managed with medical treatment. She suffered from acute spontaneous vertigo, lasting more than 24 hours without any aggravating factors initially but had recovered by the time she visited our hospital.
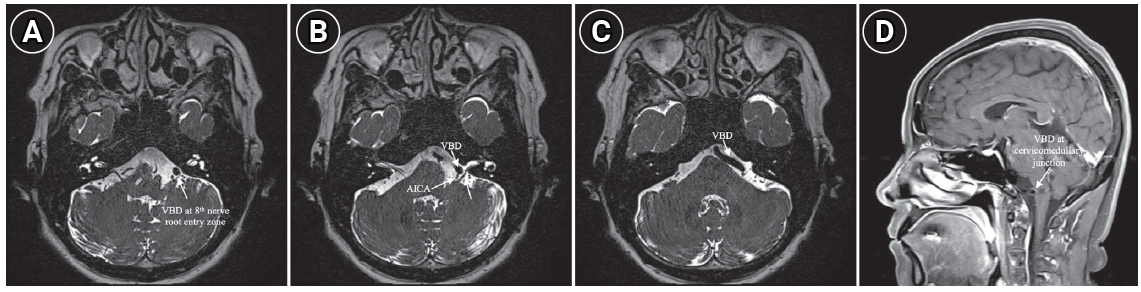
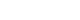
Subtle spontaneous downbeat nystagmus was observed during the visit, with no further sign of gaze-evoke directional changing nystagmus, positional nystagmus, head shaking nystagmus, and skew deviation. No other abnormal cerebellar function tests were observed. Pure tone audiometry showed a left moderate to severe sensorineural hearing loss compatible with SSNHL (pure tone audiometry, 53 dB on the left ear and 27 dB on the right ear). The threshold of speech audiometry was measured to be 55 dB on the left ear and 30 dB on the right side (Fig. 1A). The patient was diagnosed with SSNHL with vertigo, prompting the need for an MRI scan to differentiate between central and peripheral causes. The MRI showed a tortuous and dilated right vertebrobasilar artery, compressing brainstem and left 8th cranial nerve root entry zone (Fig. 2A-C) without acute cerebellar stroke. Additionally, VBD was also compressing the root segment of the left anterior inferior cerebellar artery (AICA) (Fig. 2C) and cervicomedullary junction (Fig. 2D) which originated from the affected vertebrobasilar artery. Because the 8th cranial nerve and AICA was the parent artery of the internal auditory artery, we speculated that this abnormal vascular morphology was responsible for the SSNHL with vertigo. Vestibular function tests including video head impulse test, bithermal caloric test, cervical and ocular vestibular evoked myogenic potentials, and computerized dynamic posturography showed predefined normal range results. High-dose steroid treatment was started for the SSNHL. VBD was monitored with neurosurgical consultation and determined to be observed due to the absence of obvious neurological symptoms. Her auditory symptoms and vertigo gradually improved, and the spontaneous downbeat nystagmus ceased by the third day. After three months of treatment, her left hearing has improved to 28 dB (Fig. 1B). She is still doing outpatient follow-up and has reported no dizziness or neurological issues.
DISCUSSION
VBD is the abnormal enlargement, elongation, and tortuosity of the vertbrobasilar artery, with an estimated prevalence of 0.05% to 0.1 % [3]. The disease is thought to be caused by an imbalance in the activity of metalloproteinases and proteinases in the connective tissue of the arterial walls, leading to abnormal vascular remodeling and defective formation of the connective tissue in the arterial walls [3]. VBD can lead to compression of cranial nerves originating from the brainstem resulting from an enlarged vertebrobasilar artery. This compression can result in a variety of symptoms, which include dizziness and hearing loss, in cases where the 8th cranial nerve is compromised [2,5,7]. The research indicates that audiovestibular symptoms such as hearing loss and dizziness caused by VBD might vary in severity and are reported to be rare in the form of SSNHL with vertigo [7].
There is a disagreement on how VBD can lead to audiovestibular symptoms, with the primary theory suggesting compression of the 8th cranial nerve and impaired AICA circulation [7]. The compression mechanism is that the elongated and tortuous arteries can directly displace the surrounding tissues, including CNs and the brainstem, leading to symptoms that resemble those of a CPA tumor [8]. The following mechanisms for labyrinthine ischemia in VBD are proposed as follows: (1) turbulent flow can occur between forward lamina flow within dilated artery, creating conditions for thrombus formation [12], (2) elongation and angulation of the posterior circulation artery, which can cause distortion at the entrance of the arterial branches, resulting in reduced blood flow, and (3) mechanisms by which atherosclerotic plaques secondary to endothelial injury associated with hemodynamic changes can occlude artery [12].
In the context of VBD presenting with SSNHL, the etiology of hearing impairment is multifactorial and complex, warranting an in-depth discussion. In this case, it could be that the SSNHL with vertigo was triggered by an unexpected decrease in blood flow in the labyrinthine artery due to the widening of the vertebrobasilar artery or the formation of a thrombus by the turbulent flow. The neurologic mechanism for the spontaneous downbeat nystagmus, in this case, is unclear, but it is speculated to be caused by compression of the cervicomedullary junction as the VBD compresses the lower part of the brainstem (Fig. 2D) [4,13]. This has been explained by the disappearance of downbeat nystagmus in the case of Arnold-Chiari malformation when the pressure on the brainstem or vestibular cerebellum is removed by surgical decompression [14]. However, the preservation of vestibular function in some cases, as observed in our report, suggests a selective vulnerability or resilience of these structures to vascular insufficiency, which remains an area of ongoing investigation. Another aspect to consider is the inflammatory response associated with vascular anomalies, which might play a role in the etiology of SSNHL in VBD patients. The dramatic response of SSNHL to steroids in our case suggests an underlying inflammatory component, possibly secondary to selective vascular infarction or thrombosis. This observation aligns with the broader literature on SSNHL, where steroid responsiveness often indicates an inflammatory etiology. Finally, the possibility that SSNHL in the context of VBD could be a coincidental finding. We acknowledge the possibility that VBD could be a bystander in the pathogenesis of SSNHL in this case. However, the lack of other identifiable causes for SSNHL, we propose that VBD, through its effects on vascular integrity and potential compression, contributed to the clinical presentation in this case.
Due to the absence of validated randomized controlled trials [15], there is currently a lack of high-level treatment guidelines for VBD. Conservative management is suggested for symptomatic VBD, with brain imaging follow-up at 6 months and 1 year. If the diameter of the VBD is 10 mm or larger, or if it increases by 2 mm or more within 1 year, or if new symptoms develop, microvascular decompression or endovascular treatment is recommended [15]. In this case, the 8th cranial nerve compression led to SSNHL without any further complications like cerebral infarction, hemorrhage, or hydrocephalus. Therefore, it was decided that conservative treatment involving blood pressure management and regular follow-up was suitable. It is also thought that patients with VBD may develop audiovestibular symptoms [7] over time and will need continued follow-up.
This case report demonstrates a VBD with a diameter exceeding 6 mm and significant tortuosity, causing direct compression on the brainstem and the root of the 8th cranial nerve, resulting in SSNHL with vertigo accompanying spontaneous downbeat nystagmus. The etiology of SSNHL with vertigo in patients with VBD is likely multifaceted, involving selective vascular infarction/thrombosis, direct nerve compression, and potentially an inflammatory response. Imaging studies can help determine the underlying cause of unilateral SSNHL in clinical practice. Clinicians should consider the possibility of vascular inner ear disorders in individuals experiencing SSNHL. Emphasize the use of an interdisciplinary approach for diagnosing and treating this rare disease.
ARTICLE INFORMATION
-
Funding/Support
None.
-
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
-
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article. For other data, these may be requested through the corresponding author.
-
Authors' Contributions
Conceptualization: YJL, GSN; Data curation, Investigation: WYB; Formal analysis, Methodology, Software: YJL; Funding acquisition, Project administration, Resources, Supervision, Validation: GSN; Visualization: YJL, WYB; Writing–original draft: YJL, GSN; Writing–review & editing: all authors.
All authors read and approved the final manuscript.
Fig. 1.Initial and final pure tone audiometry. (A) The initial audiometry shows a pattern of left low-frequency sudden sensorineural hearing loss. (B) The 3-month follow-up audiometry shows remarkable recovery in the left ear. AC, air conduction; BC, bone conduction, PTA, pure tone audiometry; SRT, speech recognition threshold; MCL, most comfortable level; UCL, uncomfortable loudness level.
Fig. 2.Magnetic resonance imaging findings of patient (A–C). VBD passes through the root entry zone of the 8th cranial nerve (A, C), with compressing AICA (B) and cervicomedullary junction (D), the finding which is responsible for sudden sensorineural hearing loss with vertigo. VBD, vertebrobasilar dolichoectasia; AICA, anterior inferior cerebellar artery.
REFERENCES
- 1. Flemming KD, Wiebers DO, Brown RD Jr, et al. The natural history of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis 2005;20:270–279.ArticlePubMedPDF
- 2. Anízar Rodríguez CB, Mendoza Ugalde DM, García-Tecpa RA. Case Report: mixed-cause vertigo and sudden sensorineural hearing loss as presentations of vertebrobasilar dolichoectasia. Cureus 2022;14:e28136. PubMedPMC
- 3. Prasad SN, Singh V, Selvamurugan V, Phadke RV. Vertebrobasilar dolichoectasia with typical radiological features. BMJ Case Rep 2021;14:e239866. ArticlePubMedPMC
- 4. Himi T, Kataura A, Tokuda S, Sumi Y, Kamiyama K, Shitamichi M. Downbeat nystagmus with compression of the medulla oblongata by the dolichoectatic vertebral arteries. Am J Otol 1995;16:377–381.PubMed
- 5. Murphy TP. Macrovascular sensorineural hearing loss. Am J Otol 1991;12:88–92.PubMed
- 6. Kim P, Ishijima B, Takahashi H, Shimizu H, Yokochi M. Hemiparesis caused by vertebral artery compression of the medulla oblongata. Case report. J Neurosurg 1985;62:425–429.PubMed
- 7. Frosolini A, Fantin F, Caragli V, et al. Vertebrobasilar and basilar dolichoectasia causing audio-vestibular manifestations: a case series with a brief literature review. Diagnostics (Basel) 2023;13:1750. ArticlePubMedPMC
- 8. Passero SG, Rossi S. Natural history of vertebrobasilar dolichoectasia. Neurology 2008;70:66–72.ArticlePubMed
- 9. Chandrasekhar SS, Tsai Do BS, Schwartz SR, et al. Clinical Practice Guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg 2019;161(1 Suppl):S1–S45.ArticlePubMedPDF
- 10. Shilo S, Gilboa D, Oron Y, et al. Vertebrobasilar system laterality and idiopathic sudden sensorineural hearing loss. Audiol Neurootol 2023;Oct 20 [Epub]. https://doi.org/10.1159/000534153.Article
- 11. Maruyama A, Kawashima Y, Fujikawa T, et al. Potential confounding factors may influence the association between configurations of the vertebrobasilar system and the incidence of idiopathic sudden sensorineural hearing loss and canal paresis. Otol Neurotol 2020;41:e548–e555.ArticlePubMed
- 12. Lou M, Caplan LR. Vertebrobasilar dilatative arteriopathy (dolichoectasia). Ann N Y Acad Sci 2010;1184:121–133.ArticlePubMed
- 13. Jacobson DM, Corbett JJ. Downbeat nystagmus associated with dolichoectasia of the vertebrobasilar artery. Arch Neurol 1989;46:1005–1008.ArticlePubMed
- 14. Cogan DG. Down-beat nystagmus. Arch Ophthalmol 1968;80:757–768.ArticlePubMed
- 15. Pico F, Labreuche J, Amarenco P. Pathophysiology, presentation, prognosis, and management of intracranial arterial dolichoectasia. Lancet Neurol 2015;14:833–845.ArticlePubMed
Citations
Citations to this article as recorded by

 , Wonyong Baek
, Wonyong Baek , Gi-Sung Nam
, Gi-Sung Nam




 KBS
KBS
 PubReader
PubReader ePub Link
ePub Link Cite
Cite